Guidance Development and Revision
Following the experience gained in responding to the emergence of the Severe Acute Respiratory Syndrome (SARS) in 2003 and the global spread of the avian influenza A (H5N1) virus that began in 2003, the U.S. government initiated a process to develop guidance in a number of areas for the public and private sectors to prepare for an influenza pandemic.
The initial draft of this vaccine allocation guidance was published in 2008 by a federal interagency working group whose members represented all sectors of the government. Their approach was based on the best scientific information available at that time (e.g., historical data, vaccine supply, vaccine impact in different scenarios, and different population groups), and directly considered the values of our society and the ethical issues involved in planning a phased approach to pandemic vaccination that, by necessity, means that some people will receive vaccine before others. Information considered by the working group included assessments of candidate pandemic vaccines, national and homeland security issues, essential community services and the infrastructure and workforce critical to maintaining them, and the perspectives of state and local public health and homeland security experts. Historical analysis of the influenza pandemics of 1918, 1957, and 1968 and their impacts also provided valuable insights to this guidance.
In addition to key scientific information, meetings with the public and stakeholders, including businesses and community organizations, provided key perspectives on public values and priorities about which subgroups would require the earliest vaccine protection in the event of an influenza pandemic. Meeting participants discussed and rated the importance of potential vaccination program objectives based on a severe pandemic scenario. A key principle established by the working group and reinforced by public and stakeholder input pdf icon[269, KB, 34 pages]external icon was that the goals to reduce illness and associated disruption to society and the economy, “cannot be achieved by targeting vaccine to one occupational or risk group at the exclusion of others.” Notably, pandemic planning working groups, public engagement meetings, and stakeholder meetings came to the same conclusions about which program objectives were the most important:
- Protecting those who are essential to the pandemic response and provide care for persons who are ill,
- Protecting those who maintain national security and essential community services,
- Protecting children and pregnant women, and
- Protecting workers who are at greater risk of infection due to their job.
Working group discussions also highlighted the important federal objective of maintaining homeland and national security.

General Principles and Interim Guidance on Pandemic Vaccination (Established in 2008)
- At the time of the next pandemic, CDC, with input from across the U.S. Department of Health and Human Services (HHS), U.S. Department of Homeland Security (DHS), other relevant federal agencies will:
- assess pandemic severity,
- determine whether the pandemic is likely to result in significant and disruptive workplace absenteeism (especially among critical workforce),
- advise whether vaccine targeting guidance should be used, and if so, how it should be modified based on the characteristics of the emerging pandemic and pandemic vaccine availability, and
- assess vaccine in the federal stockpile to determine whether it can be used to reduce the impact of the pandemic.
- The need to target vaccine to maintain national security, health care, and other essential community services and to preserve critical infrastructure will depend on the severity of the pandemic and vaccine availability, as rates of absenteeism and the ability to supply essential products and services will differ for more or less severe pandemics. As a result, groups identified for earlier vaccination will differ depending on pandemic severity and vaccine supply.
- Allocation of pandemic vaccines by the U.S. government to states and territories will likely be in proportion to states and territories’ populations.
- States should follow the national guidance to ensure fairness and uniformity across the U.S. and minimize confusion, as much as possible. Within the parameters of the guidance, states will have the authority to approve vaccine orders from providers in their jurisdiction in order to distribute vaccine to meet the specific needs in their populations. In past pandemics, groups at increased risk for serious illness and death have differed by age and health status. Because the high-risk groups in the next pandemic are not known in advance, planners should consider how the guidance might be modified for different pandemic scenarios (Implementation of this guidance during a future pandemic).
- Guidance on pandemic vaccine allocation and targeting will be reassessed periodically before and throughout a pandemic to consider new scientific information, including risk of severe outcomes by age and risk groups, changes in vaccine production capacity, and advances in other health and public health response measures.
This revised guidance updates the prior 2008 guidance with the following major changes:
- Updates severity categories based on the current CDC Pandemic Severity Assessment Framework2.
- Incorporates lessons learned from the 2009 H1N1 pandemic response, such as the unpredictability of pandemic severity and timing, variability of the impact of pandemic severity on critical infrastructure functions, challenges with vaccine supply overall and variability among manufacturers, and the need for flexibility at the state, tribal and local levels to best manage vaccine supplies to meet local needs.
- Includes the consideration that two doses of vaccine and co-administration of adjuvant may be required to produce protective immunity in some scenarios.
- Moves pharmacists and pharmacy technicians to Tier 1, since pharmacists and pharmacy technicians will be crucial to antiviral dispensing, and many pharmacists will be pandemic vaccine immunizers.
- Updates U.S. population numbers based on the 2005 U.S. population of 300 million to the population estimates from the 2015 U.S. Census (321 million)3.
Input from multiple sectors and public health officials also informed this revision. Importantly, however, the 2009 H1N1 pandemic and subsequent discussions with stakeholders reinforced the core objectives and guiding principles established previously with stakeholders, and preserved in this document.
The guidance also includes consideration for allocating stockpiled pre-pandemic vaccine (i.e., vaccine produced against novel influenza A viruses with pandemic potential), if stockpiled vaccine is likely to be effective against the pandemic virus. In addition, the guidance now includes the consideration that two doses of vaccine and co-administration of adjuvant may be required to produce protective immunity in some scenarios.
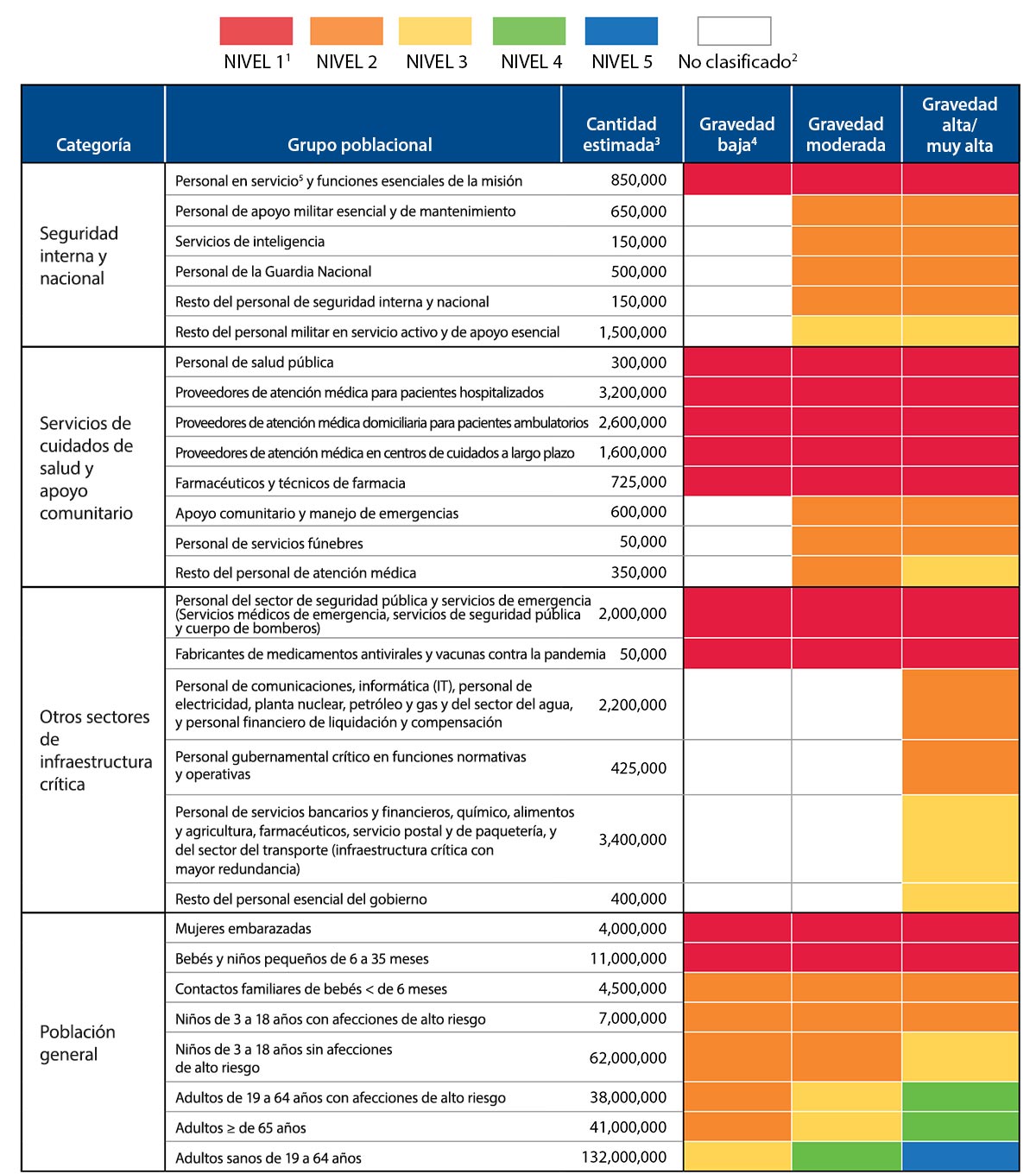
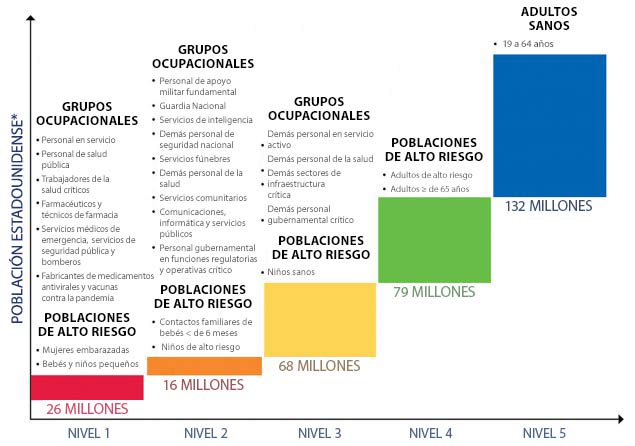
Categories – Pandemic vaccination population groups are clustered into four broad categories (homeland and national security, health care and community support services, critical infrastructure, and the general population). These four categories together cover the entire population.
Population Groups – People targeted for vaccination defined by occupation, age group, or risk level.
Tiers – Across categories, vaccine will be allocated and administered according to tiers where all groups designated for vaccination within a tier have equal priority for vaccination. Groups within tiers vary depending on pandemic severity.
Critical Workforce – Workers with critical skills, experience, certification or licensure status whose absence would create severe bottlenecks in or the collapse of critical functions.
Framework for Targeting Pandemic Influenza Vaccine
Revised guidance for allocating and targeting initial vaccination of certain groups includes a structure, as in the 2008 guidance, that defines population groups in four broad categories that correspond with the objectives of a pandemic vaccination program – to protect people who 1) maintain homeland and national security, 2) provide health care and community support services, 3) maintain critical infrastructure, and 4) are in the general population.
Each broad category includes specific populations that are defined by their occupation or by their age and health status (see Table 1) for the purpose of organizing the vaccination program and targeting of vaccination when the initial supply of vaccine is limited. As identified in Table 1, population groups are offered vaccine in “tiers” based the severity of the pandemic. Reflecting public values and the need to address multiple important objectives with the pandemic vaccination program, each of the top tiers includes populations from all four categories for a severe pandemic.
Critical workforces are more specifically targeted in a more severe pandemic than when the pandemic severity is lower and the risk to societal and community functioning is also lower. Once a novel influenza virus has emerged and is circulating in human populations, the risk posed by the epidemic itself can be assessed. Pandemic vaccine target groups will differ depending on pandemic severity and vaccine supply. In all levels of pandemic severity, public health and front-line healthcare providers, emergency services personnel, deployed and mission essential personnel; manufacturers of pandemic vaccine and antivirals; and pregnant women, infants and toddlers would be targeted for early receipt of vaccine. However, targeting of other critical workforce groups would depend on pandemic severity, the risk of severe illness by age group, vaccine supply, and the accompanying disruption to security, society, and the economy. For instance, there likely would be more focused targeting of critical workforce in a pandemic with a high or very high level of severity and limited early supply of pandemic vaccine, and less need for targeting critical workforce in less severe pandemics as threats to security, society, and the economy will be less significant in those pandemics. CDC will utilize the Pandemic Severity Assessment Framework (PSAF)1 which incorporates an assessment of influenza transmissibility and clinical severity, to determine pandemic severity. The U.S. government will use this assessment to develop more specific guidance on vaccination prioritization and determine how the tier schema outlined in this document will be adapted to an actual pandemic.
Defining Who is Included in Each Population Group
Everyone in the United States is included in at least one vaccination population group. Individuals not included in an occupational group will be vaccinated as part of the general population based on their age and health status. When a person is included in more than one population group, they will be vaccinated in the highest tier group in which they are included. Occupationally-defined groups (i.e., those defined in the homeland and national security, health care and community support services, and other critical infrastructure categories) do not include the entire workforce, but rather only persons who, based on the nature of their role or occupation, are individually critical for providing essential services during a pandemic (Appendix A). Preliminary identification of critical functions was partly based on an analysis of critical sectors and workforces conducted by DHS’s National Infrastructure Advisory Councilexternal icon (NIAC), along with input from other federal agencies. Additional planning in each community, in coordination with its state, tribes, or territory, is recommended to more specifically determine and identify the individuals in critical occupations and other potentially prioritized groups who should receive early vaccination so that employers and public health emergency planners can reach those individuals quickly and without confusion when necessary. Because an influenza pandemic differs from other national emergencies in the threat it poses and the duration over which it will affect our nation and communities, populations targeted for vaccination within each sector may be different from those defined in other emergency response plans.
It should be noted that members of occupational groups are defined by the functions that persons within that group are anticipated to perform during the pandemic; it does not distinguish among staff performing these duties as part of their usual functions, those being reassigned to perform the function as a new response role, or those performing the function as a volunteer. It should also be noted that vaccine does not replace, but adds to other measures taken to protect the workforce and general population.
The primary objective of vaccinating persons in critical infrastructure sectors is to protect workers with critical skills, experience, certification or licensure status whose absence would create severe bottlenecks in or the collapse of critical functions and to protect workers who are at especially high occupational risk (e.g., emergency services personnel and essential utility services personnel). Other pandemic response strategies (e.g., prompt treatment, asking sick workers to stay home and away from the workplace, antiviral post-exposure prophylaxis, engineering controls in workplaces, changing work practices to reduce close contact with others, good hand washing, or worker education) are likely to be effective in decreasing absenteeism and are recommended for the entire workforce, not just those fulfilling critical functions.
Guidance for Targeting Pandemic Vaccination
National guidance for targeting pandemic influenza vaccination planning is provided in Table 1 with the understanding that the numbers of people within each group are estimates and actual recommendations may change for a future pandemic and as the pandemic progresses. In general, all groups designated for vaccination within a tier have equal priority for vaccination. However, an important lesson learned from the 2009 H1N1 pandemic and other public health emergencies is that decisions at the state, local, and provider level may be appropriate to adapt these designations to local realities. State and territorial health departments, in coordination with local health departments, will be responsible for allocating vaccine to providers who agree to target vaccine to persons in the targeted groups or who care for persons in a targeted group (e.g., allocate vaccine to obstetricians to reach pregnant women). These decisions are likely to vary among states as situations and plans vary among states. During a severe influenza pandemic, the most critical workforce will likely be vaccinated in temporary closed mass vaccination clinics or in existing occupational health clinics as coordinated through the local/state health department.
Recommendations regarding those groups targeted for early vaccination are tailored to pandemic severity and transmissibility and based on vaccine supply in order to best achieve national pandemic response goals and objectives. The targeting approach will depend on the pandemic severity assessment and the risk that the pandemic poses to groups at highest risk of complications from influenza and those who maintain critical functions of society. If the severity of a pandemic is high or very high, it will also be important to emphasize measures to preserve critical societal functions. However, flexibility in planning is needed as it is not possible to predict in advance the severity of a future pandemic, or the impact that the pandemic will have on certain population sub-groups. Therefore, this pre-pandemic interim planning guidance will need to be re-assessed at the time of an emerging pandemic.
Footnote
- Tier 1 includes the highest priority population groups to receive vaccination if there is limited vaccine supply for any level of pandemic severity. See Table 1.