División de Influenza
En esta página
Our Mission
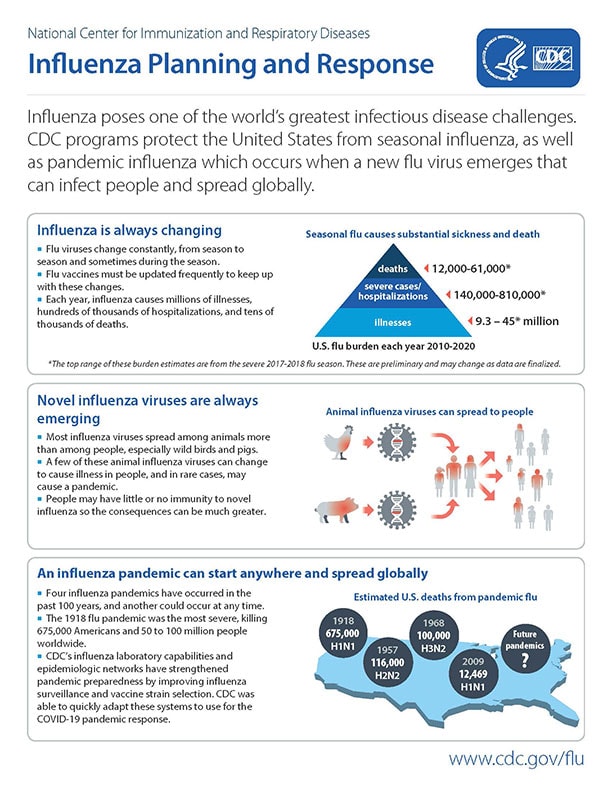
The Influenza Division (ID) improves global control and prevention of seasonal and novel influenza and improves influenza pandemic preparedness and response.
In collaboration with domestic and global partners, the ID:
- Builds surveillance and response capacity
- Monitors and assesses influenza viruses and illness Improves vaccines and other interventions
- Applies research to provide science-based enhancement of prevention and control policies and programs
For more information on influenza, please visit the seasonal flu website.
Lo que hacemos
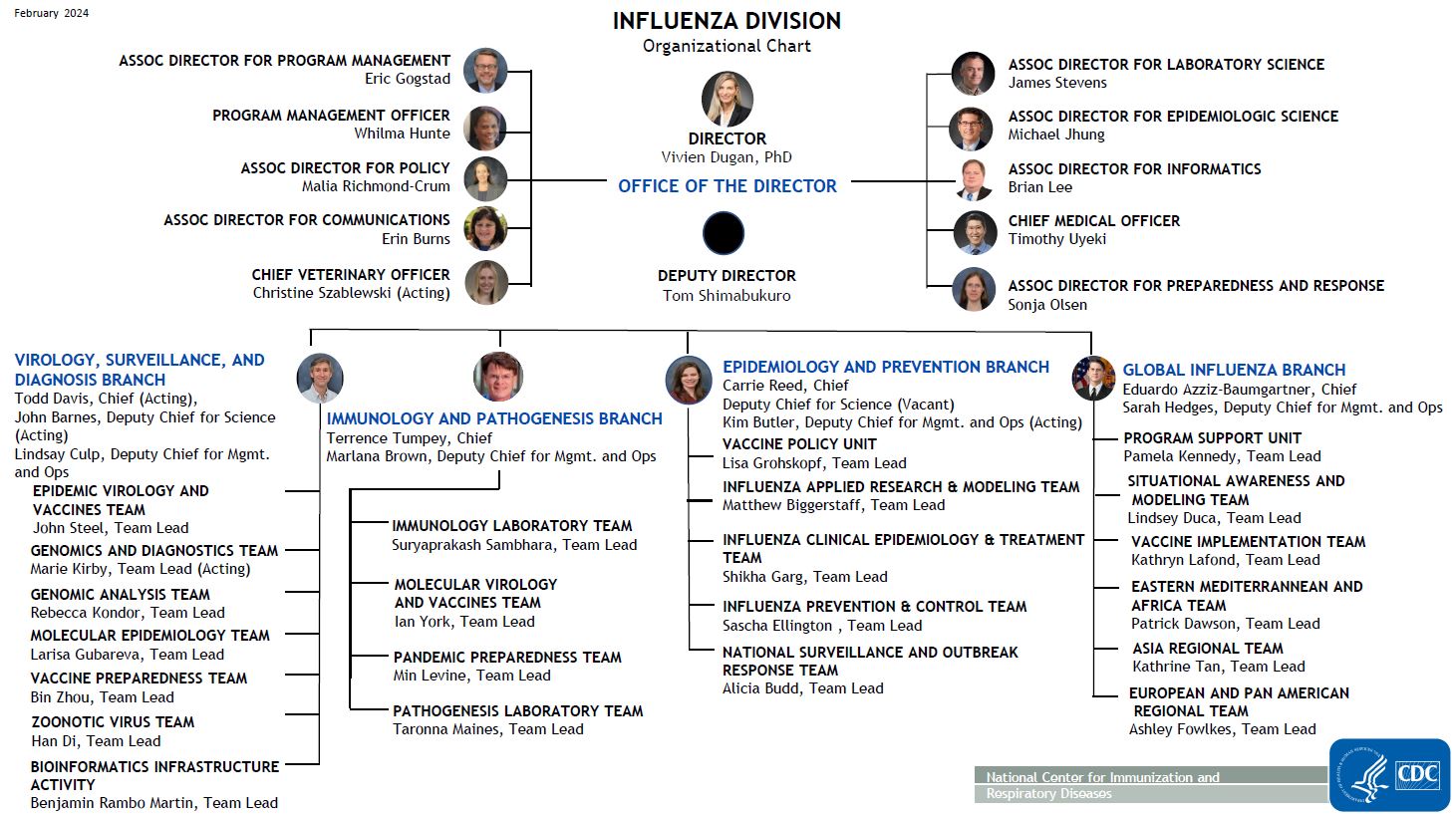
Oficina del Director
- Provides vision, leadership and direction for the Influenza Division
- Provides leadership, oversight and resources for Division-wide business functions through coordination and collaboration
- Fosters external partnerships and cross-cutting activities that support quality science and strong global partnerships
- Provides leadership in formulation of public health guidance and recomendaciones
- Provides support for national and international capacity building programs
- Provides technical expertise and leadership for national and international pandemic preparedness activities
- Provides technical expertise for communications, public health guidance, informatics, epidemiologic and laboratory science, and reagent resources
- Assesses the potential pandemic risk posed by influenza viruses that currently circulate in animals
Virology, Surveillance, and Diagnosis Branch
Todd Davis, MS, Ph.D. – Acting Branch Chief
- Advances influenza virus control, prevention and pandemic preparedness and response
- Develops and distributes diagnostics and other reagents to detect and characterize influenza viruses
- Trains and supports more than 200 domestic and international laboratories that perform influenza testing
- Conducts comprehensive antigenic, phenotypic, genotypic, and evolutionary characterization of human (epidemic/seasonal) and animal (pandemic potential) influenza viruses
- Evaluates antiviral drugs and novel therapeutics to assess the emergence of drug resistant viruses
- Performs genetic and antigenic pandemic risk assessment of novel influenza viruses
- Generates and evaluates candidate vaccine viruses for distribution to vaccine manufacturers
- Serves as the WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza
Epidemiology and Prevention Branch
Carrie Reed, D.Sc., M.P.H. – Branch Chief
- Conducts and builds capacity for national and global influenza surveillance to enhance the ability to monitor influenza viruses and associated illnesses and to detect novel influenza viruses
- Improves the understanding of the effectiveness and impact of influenza vaccines to inform vaccine policy
- Improves the understanding of risks associated with influenza infection and severe influenza in vulnerable populations
- Estimates the carga de enfermedad and severity of influenza in the U.S. population
- Leads collaborations and projects to foster innovation in influenza modeling and predecir
- Expands the use of influenza vaccines globally, especially among vulnerable populations
- Optimizes the use of influenza antiviral drugs
- Works with a wide range of international partners, including the World Health Organization and National Ministries of Health, to prevent, control and build capacity for pandemic and seasonal influenza
- Maintains international regional influenza offices to facilitate partnerships for enhancing and optimizing global influenza surveillance, outbreak investigations and pandemic preparedness
Immunology and Pathogenesis Branch
Terrence Tumpey, Ph.D. – Branch Chief
- Increases knowledge and improves the understanding of immunity and immune correlates of protection
- Develops and improves influenza vaccines
- Determines virus and host factors that impact virulence and transmission of influenza viruses
- Conducts immunologic and virologic pandemic risk assessment of novel influenza viruses
- Trains and supports domestic and international laboratories that perform immunologic testing for influenza
Global Influenza Branch
Eduardo Azziz-Baumgartner, MD, MPH – Branch Chief
- Works with a range of global partners, including the World Health Organization (WHO) and over 100 countries to detect, prevent, and strengthen capacity to respond quickly to pandemic and seasonal influenza threats
- Maintains regional influenza hubs and staff in 7 countries across WHO regions to facilitate partnerships and provide technical support for global influenza surveillance, outbreak investigations and pandemic preparedness and response
- Supports epidemiologic and virologic surveillance and reporting to WHO’s Global Influenza Surveillance and Reporting System to guide vaccine virus selection
- Enhances laboratory capacity for the timely detection and characterization of viruses with pandemic potential, as well as efficient detection of seasonal influenza viruses
- Develops and communicates the evidence-base to support local policy-decisions for global influenza control and prevention, including use of influenza vaccines and antiviral medications
- Advances global innovation in forecasting and modeling for seasonal influenza
- Leverages data modernization initiatives to strengthen surveillance of global seasonal and zoonotic influenza
- Supports improvements to seasonal influenza programs globally to reduce disease impact through:
- Vaccine introduction and evaluation
- Evaluating and expanding use of non-pharmaceutical interventions
- Evaluating the use of antiviral medications
Influenza Division Priorities
- Improve vaccine impact
- Improve influenza detection and control
- Improve epidemic and pandemic risk assessment and readiness
- Influenza Division Strategic Priorities [6 páginas]