2014-2015 Influenza Season Week 40 ending October 4, 2014
All data are preliminary and may change as more reports are received.
Background: The Centers for Disease Control and Prevention’s (CDC) Influenza Division collects and analyzes influenza surveillance data year-round and produces a weekly report on U.S. influenza activity during the influenza season which begins at week 40 each year. The U.S. influenza surveillance system provides information in five categories collected from eight data sources. This is the first report of the 2014-2015 influenza season, which began on September 28, 2014, and also summarizes influenza activity in the United States during the summer weeks of the 2013-14 season.
The five categories and eight data components of CDC influenza surveillance are:
- Viral Surveillance: U.S. World Health Organization (WHO) collaborating laboratories, the National Respiratory and Enteric Virus Surveillance System (NREVSS), and human infection with novel influenza A virus case reporting;
- Mortality: 122 Cities Mortality Reporting System and influenza-associated pediatric deaths;
- Hospitalizations: Influenza Hospitalization Network (FluSurv-NET) including the Emerging Infections Program (EIP),
- Outpatient Illness Surveillance: U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet);
- Geographic Spread of Influenza: State and territorial epidemiologists’ reports.
In addition to the eight data components described above, for the 2014-2015 influenza season, the use of National Center for Health Statistics (NCHS) pneumonia and influenza mortality surveillance data for the rapid assessment of influenza-associated mortality will be piloted. An overview of influenza surveillance, including a description of the NCHS mortality surveillance data, is available at http://www.cdc.gov/flu/weekly/overview.htm
Synopsis:
During week 40 (September 28-October 4, 2014), influenza activity was low in the United States.
- Viral Surveillance: Of 6,192 specimens tested and reported by U.S. World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories during week 40, 199 (3.2%) were positive for influenza.
- Pneumonia and Influenza Mortality:The proportion of deaths attributed to pneumonia and influenza (P&I) was below the epidemic threshold.
- Influenza-associated Pediatric Deaths: No influenza-associated pediatric deaths were reported.
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 1.3%, which is below the national baseline of 2.0%. All 10 regions reported ILI below region-specific baseline levels. Puerto Rico experienced high ILI activity, all 50 states and New York City experienced minimal ILI activity and the District of Columbia had insufficient data.
- Geographic Spread of Influenza: The geographic spread of influenza in Guam was reported as widespread; Puerto Rico reported regional activity; three states reported local activity; 28 states, the District of Columbia, and the U.S. Virgin Islands reported sporadic activity; 18 states reported no influenza activity; and one state did not report.
| HHS Surveillance Regions* | Data for week 40 (September 28-October 4, 2014) | |||||||
|---|---|---|---|---|---|---|---|---|
| Out-patient ILI† | % positive for flu‡ | Number of jurisdictions reporting regional or widespread activity§ | 2009 H1N1 | A (H3) | A(Subtyping not performed) | B | Pediatric Deaths | |
| Nation | Normal | 3.2% | 2 of 54 | 0 | 32 | 90 | 77 | 0 |
| Region 1 | Normal | 1.3% | 0 of 6 | 0 | 2 | 6 | 1 | 0 |
| Region 2 | Normal | 0.8% | 1 of 4 | 0 | 0 | 0 | 3 | 0 |
| Region 3 | Normal | 1.2% | 0 of 6 | 0 | 3 | 2 | 2 | 0 |
| Region 4 | Normal | 7.0% | 0 of 8 | 0 | 9 | 61 | 57 | 0 |
| Region 5 | Normal | 2.0% | 0 of 6 | 0 | 4 | 5 | 0 | 0 |
| Region 6 | Normal | 2.6% | 0 of 5 | 0 | 7 | 3 | 8 | 0 |
| Region 7 | Normal | 0.7% | 0 of 4 | 0 | 0 | 6 | 2 | 0 |
| Region 8 | Normal | 0.9% | 0 of 6 | 0 | 3 | 1 | 3 | 0 |
| Region 9 | Normal | 5.4% | 1 of 5 | 0 | 2 | 5 | 0 | 0 |
| Region 10 | Normal | 1.8% | 0 of 4 | 0 | 2 | 1 | 1 | 0 |
*HHS regions (Region 1 CT, ME, MA, NH, RI, VT; Region 2: NJ, NY, Puerto Rico, US Virgin Islands; Region 3: DE, DC, MD, PA, VA, WV; Region 4: AL, FL, GA, KY, MS, NC, SC, TN; Region 5: IL, IN, MI, MN, OH, WI; Region 6: AR, LA, NM, OK, TX; Region 7: IA, KS, MO, NE; Region 8: CO, MT, ND, SD, UT, WY; Region 9: AZ, CA, Guam, HI, NV; and Region 10: AK, ID, OR, WA).
† Elevated means the % of visits for ILI is at or above the national or region-specific baseline
‡ National data are for current week; regional data are for the most recent three weeks
§ Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
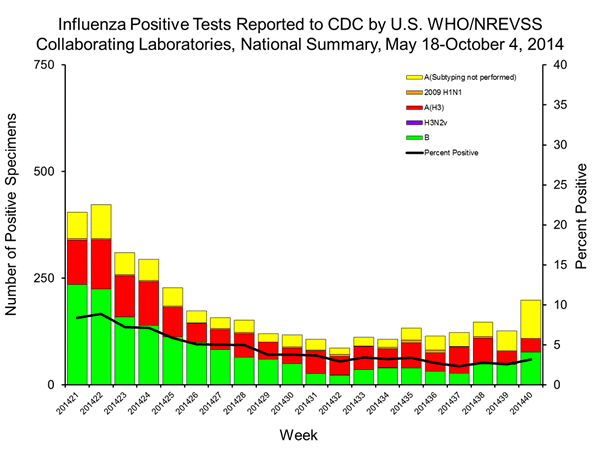
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories located in all 50 states, Puerto Rico, and the District of Columbia report to CDC the number of respiratory specimens tested for influenza and the number positive by influenza virus type and influenza A virus subtype. The results of tests performed during the current week are summarized in the table below. Region specific data are available at http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html.
Starting during week 40 (week ending October 4, 2014) of the 2014-2015 season, virologic surveillance data from approximately 120 additional NREVSS laboratories will be added to the national influenza laboratory surveillance system. Please note that historical data from these additional NREVSS laboratories will not be added to previous influenza seasons, including the 2013-2014 season.
| Week 40 | |
|---|---|
| No. of specimens tested | 6,192 |
| No. of positive specimens (%) | 199 (3.2%) |
| Positive specimens by type/subtype | |
| Influenza A | 122 (61.3%) |
| 2009 H1N1 | 0 (0%) |
| H3 | 32 (26.2%) |
| Subytping not performed | 90 (73.8%) |
| Influenza B | 77 (38.7%) |
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
Novel Influenza A Viruses:
No novel influenza A virus infections were reported to CDC during week 40, however, from May 18 through September 27, 2014, two human infections with influenza A (H3N2) variant viruses were reported by Ohio. More information is available at http://www.cdc.gov/flu/swineflu/h3n2v-case-count.htm.
Antigenic Characterization:
No antigenic characterization data is available for specimens collected after October 1, 2014.
During May 18 – September 27, 2014, CDC antigenically characterized 225 viruses collected from the United States, including six pH1N1 viruses, 93 influenza A (H3N2) viruses, and 126 influenza B viruses. All six (100%) pH1N1 viruses were antigenically similar to A/California/7/2009, the influenza A (H1N1) component of the 2014-2015 Northern Hemisphere influenza vaccine. Of the 93 influenza A (H3N2) viruses characterized, 39 (42%) were antigenically similar to A/Texas/50/2012, the influenza A (H3N2) component of the 2014-2015 Northern Hemisphere influenza vaccine.
Of the 126 influenza B viruses collected and analyzed during this period, 95 (75%) belonged to the B/Yamagata-lineage, and were antigenically similar to the B/Massachusetts/2/2012 virus, the influenza B component for the 2014–2015 Northern Hemisphere trivalent vaccine. The remaining 31 viruses (25%) belonged to the B/Victoria lineage and were antigenically similar to the B/Brisbane/60/2008 virus, the B/Victoria-lineage component of the 2014–2015 Northern Hemisphere quadrivalent influenza vaccine.
Antiviral Resistance:
No antiviral resistance data is available for specimens collected after October 1, 2014. During May 18 – September 27, 2014, 229 specimens (six 2009 H1N1, 113 influenza A (H3N2), and 110 influenza B viruses) collected in the United States were tested for susceptibility to the neuraminidase inhibitors (oseltamivir and zanamivir), none of the tested viruses were found to be resistant to either oseltamivir or zanamivir.
The majority of currently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir and zanamivir; however, rare sporadic instances of oseltamivir-resistant 2009 H1N1 and A (H3N2) viruses have been detected worldwide. Antiviral treatment with oseltamivir or zanamivir is recommended as early as possible for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.
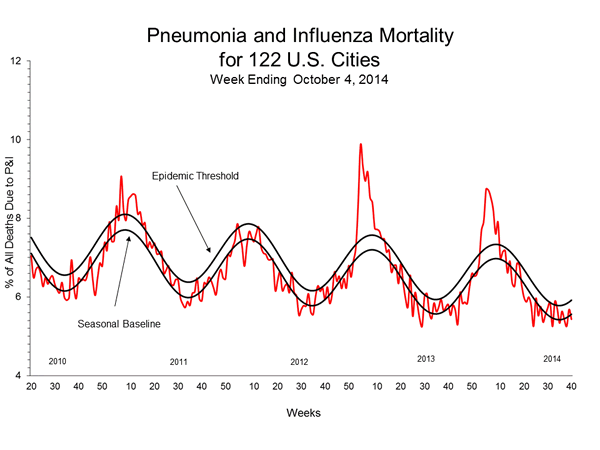
Pneumonia and Influenza (P&I) Mortality Surveillance:
During week 40, 5.4% of all deaths reported through the 122 Cities Mortality Reporting System were due to P&I. This percentage was below the epidemic threshold of 6.0% for week 40.
View Full Screen | View PowerPoint Presentation
For the 2014-2015 influenza season, CDC/Influenza Division and the National Center for Health Statistics (NCHS) are collaborating on a pilot project to use NCHS mortality surveillance data for the rapid assessment of pneumonia and influenza (P&I) mortality. To view the data, please click here (http://www.cdc.gov/flu/weekly/nchs.htm).
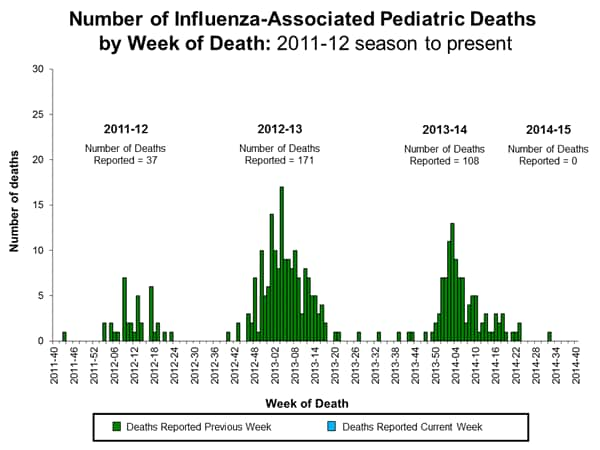
Influenza-Associated Pediatric Mortality:
No influenza-associated pediatric deaths were reported to CDC during week 40. However, five influenza-associated pediatric deaths occurring during May 18-September 27, 2014 were reported. Two deaths were associated with an influenza A (H3N2) virus, one was associated with an influenza A virus for which no subtyping was performed, and two were associated with an influenza B virus. A total of 108 influenza-associated pediatric deaths have been reported during the 2013-2014 season.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts all age population-based surveillance for laboratory-confirmed influenza-related hospitalizations in select counties in the Emerging Infections Program (EIP) states and Influenza Hospitalization Surveillance Project (IHSP) states. FluSurv-NET estimated hospitalization rates will be updated weekly starting later this season. Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
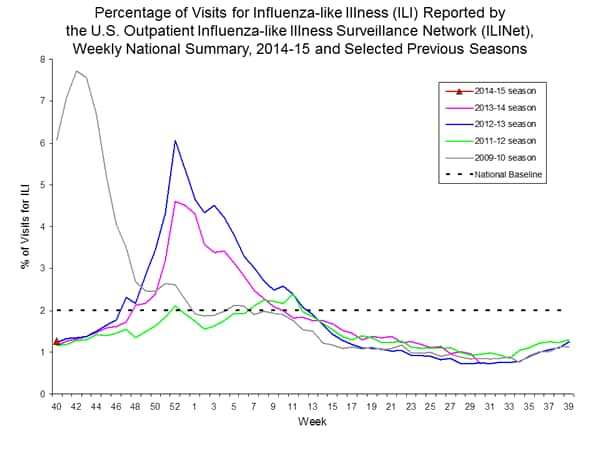
Outpatient Illness Surveillance:
Nationwide during week 40, 1.3% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is below the national baseline of 2.0%.
(ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.)
Additional data are available at http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html.
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
On a regional level, the percentage of outpatient visits for ILI ranged from 0.5% to 2.1% during week 40. All 10 regions reported a proportion of outpatient visits for ILI below their region-specific baseline levels.
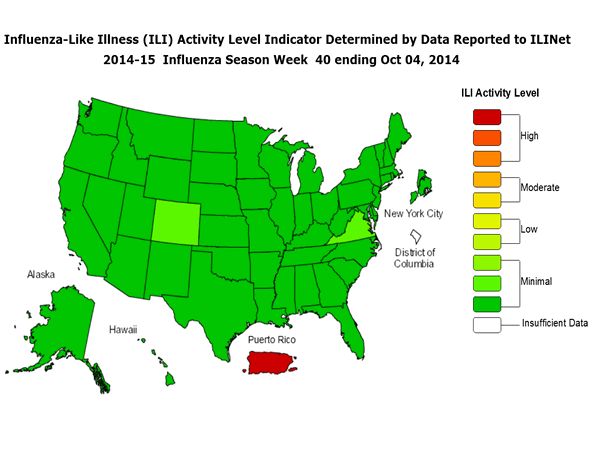
ILINet State Activity Indicator Map:
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below, or only slightly above, the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 40, the following ILI activity levels were experienced:
- Puerto Rico experienced high ILI activity.
- All 50 states and New York City experienced minimal ILI activity.
- Data were insufficient to calculate an ILI activity level from the District of Columbia.
*This map uses the proportion of outpatient visits to health care providers for influenza-like illness to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map is based on reports from state and territorial epidemiologists. The data presented in this map is preliminary and may change as more data is received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
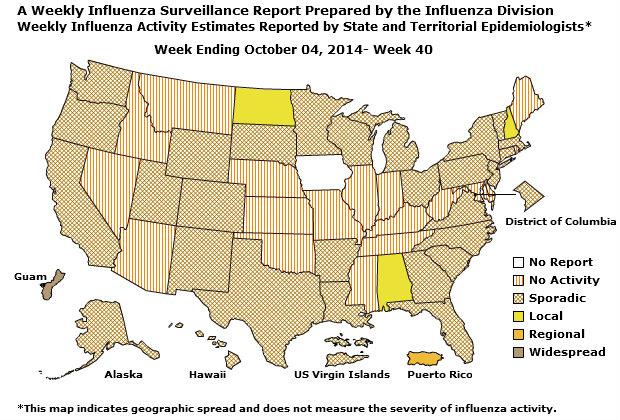
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
During week 40, the following influenza activity was reported:
- Widespread influenza activity was reported by Guam.
- Regional influenza activity was reported by Puerto Rico.
- Local influenza activity was reported by three states (Alabama, New Hampshire, and North Dakota).
- Sporadic influenza activity was reported by the District of Columbia, the U.S. Virgin Islands, and 28 states (Alaska, Arkansas, California, Colorado, Connecticut, Florida, Georgia, Hawaii, Louisiana, Massachusetts, Michigan, Minnesota, New Jersey, New Mexico, New York, North Carolina, Ohio, Oregon, Pennsylvania, South Carolina, South Dakota, Texas, Utah, Vermont, Virginia, Washington, Wisconsin, and Wyoming).
- No influenza activity was reported by 18 states (Arizona, Delaware, Idaho, Illinois, Indiana, Kansas, Kentucky, Maryland, Maine, Mississippi, Missouri, Montana, Nebraska, Nevada, Oklahoma, Rhode Island, Tennessee, and West Virginia).
- One state (Iowa) did not report.